The Process
From 2015, geneticists have been working on a new research study that will see the safe conception of three-parent babies worldwide. Research studies have been especially intense within the United Kingdom.
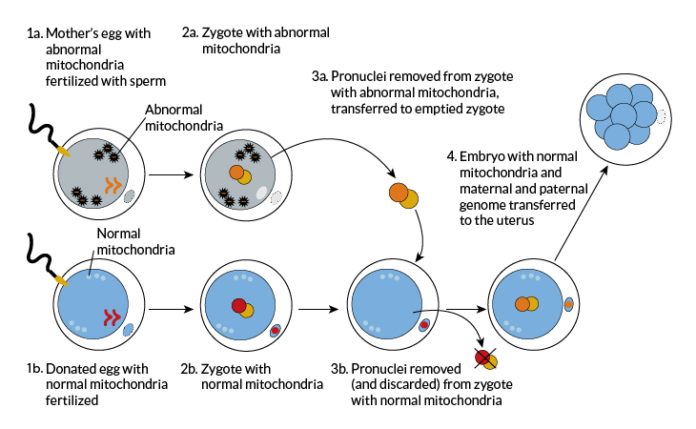
The Mitochondrial Reproductive Therapy procedure is an in-vitro fertilization (IVF) process involves three DNA strains, that is, from the father, the mother and the egg donor. The method aims to reduce the risk of mothers with mitochondrial diseases passing on their condition onto their babies. Mitochondrial diseases are those which affect the ability of the mitochondria, the organelles that convert oxygen and food into energy within the body, to do so. Conditions that hinder the optimal functionality of the mitochondria may result in weakened tissue throughout an individual’s body. Organs such as the brain, heart, muscles and the kidney may fail due to such diseases.
The procedure occurs in three steps. In the first stage, the nucleus of the mother’s fertilized egg is surgically removed and stored in the fertility clinic. The second step involves removing the nucleus of the donor’s egg. The nucleus obtained from the second stage is destroyed unlike that gotten from the first stage. Stage three of the three-parent baby IVF process involves the nucleus from the mother’s egg being inserted into the donor egg to produce a sustainable embryo. The mother can then receive the egg after the third stage. This embryo will have three strings of DNA on the completion of this procedure. Alternatively, a parent may opt to use her eggs even if the mother suffers from mitochondrial disorders. The mother’s egg will be removed and taken through a screening process. If the egg shows little risk of transmission of the disease between mother and the future child, the fertility specialist involved will give the go-ahead for the embryo to be re-inserted.
Treatment
The MRT was approved for use in fertility clinics by the Human Fertilization and Embryology Authority (HFEA) in mid-December 2016. The HFEA is the body in charge of verifying the validity of fertility studies and the ethics of the fertility clinics. “The approval of this study comes as a result of myriads of studies conducted by researchers in the field,” remarked Sally Cheshire, the chair of the HFEA.
Fertility clinics will apply for licenses from the HFEA to offer the procedure in their general treatment plans or for particular couples. Each case will be judged on its merit and approval will be issued to cases that demonstrate a real need for the procedure.
Already more than twenty couples have undergone the MRT, and some of them have babies due within the first months of 2017.
With the approval of the MRT, mothers with mitochondrial diseases can now have children with lowered risk of passing on their conditions to their progeny. Further research is being carried out to obliterate this risk factor, and some studies are even aiming at improving embryo quality. The approval of the MRT procedure is the first step toward this.
The treatment will be available at the Newcastle Fertility Center from this summer.